Looking for support on your journey to market authorization and commercialization?
We can help.
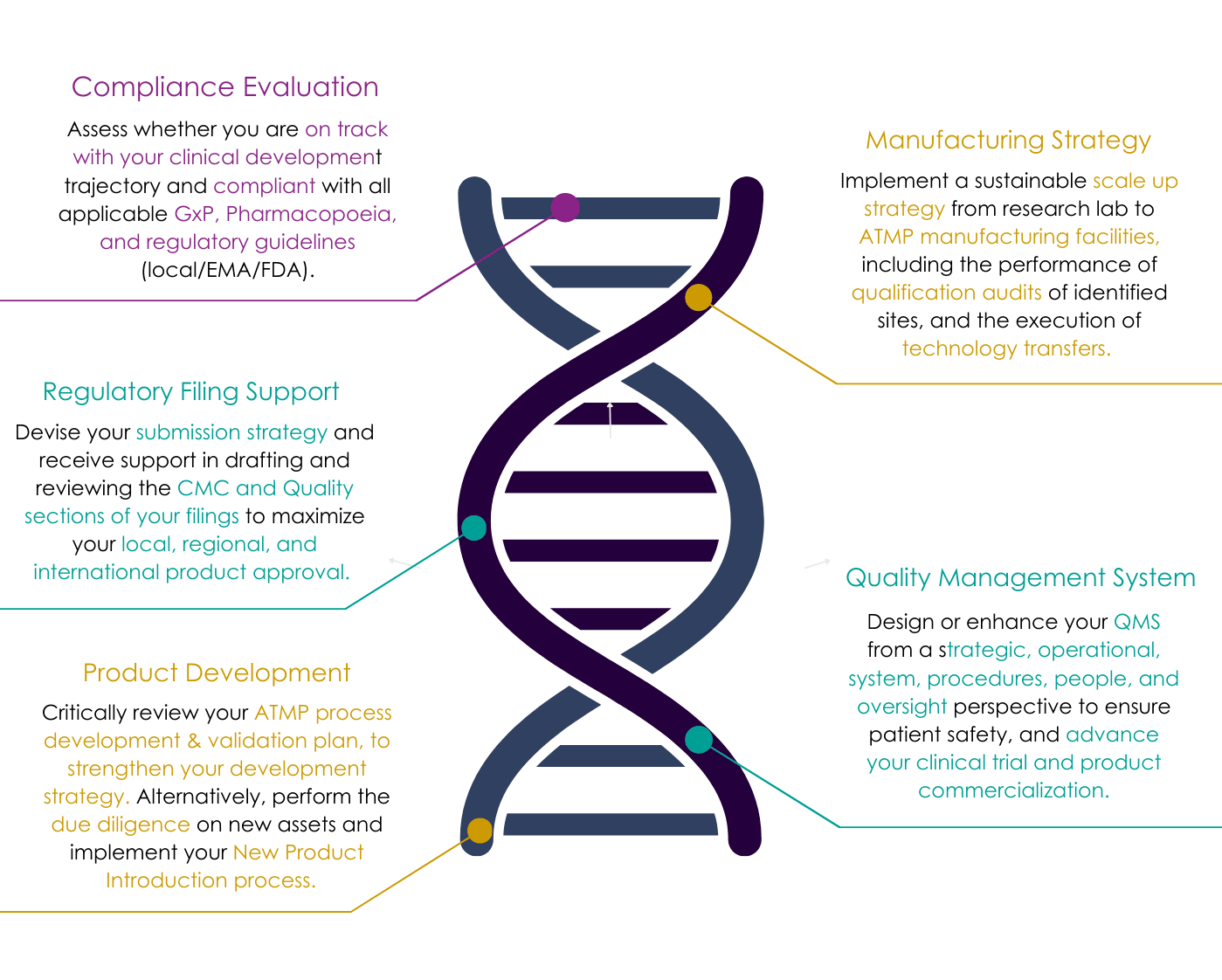
Available Now
We have a unique opportunity to offer our fully-formed, multidisciplinary Quality Assurance Operations and Compliance Team fluent in ATMP, Cell and Gene Therapy.
They are ready to support you immediately in your current phase of development to drive your success from pre-clinical, to clinical development, through to product commercialization.
Meet Your Cohesive Quality Assurance Team

Reinout Hesselink
- Sr. Quality expert in CGT manufacturing
- Qualified auditor
- Regulatory consultant

Jasbir Rattu
- Certified QP, Auditor and Trainer for ATMPs and Biologicals
- ATMP/Biologicals design, CMC development, Quality and GMP expert

Özlem Kocaağaoğlu
- Project support and logistics

Britt Dam Raaby
- Compliance
- QMS Implementation
- Process Development

Vanessa Döring
- Project management and Main Point of Contact

Kieran Canisius
- Executive Project Oversight

Claudia Horneck
- eClinical System Management
- QMS System set-up and Computer Systems Validation (CSV)
Testimonials
Our Webinars
Your Burning CRO and Vendor Selection Questions… Answered
In this virtual webinar, we harness the analytical power of Google and take a look at the most frequently searched CRO and Vendor selection questions asked by life science professionals like yourself.
5 Best Practices in CRO Selection for Biotech
In this webinar our CEO, Kieran shares actionable insights about selecting the best fit CRO for your biotech. You’ll learn how to infuse these best practices that drive success in your clinical trial including, nurturing a common purpose culture, honesty, and compliance.
Interested to speaking with one of our life science consultants?
At Seuss+, we support, inspire and challenge each other every day. No matter the project, client or task, together, we’ll boldly tackle our industries biggest challenges.



