Your Hands-On Partner For Clinical Development Success
Supporting small to mid-sized biotech and pharmaceutical companies that need more than advice—providing hands-on expertise.Who We Help: Biotech and Pharma in Clinical Development
As a sponsor, you need more than guidance; you need a partner who works directly with you to manage the complexity of clinical trials. With over 13 years of experience, Seuss+ provides hands-on support to navigate operational, regulatory, and vendor-related challenges, ensuring trials stay on time, on budget, and fully compliant with global regulations.
We don’t just advise—we integrate into your operations, managing vendors, financial oversight, and risk mitigation to support each phase of your trial.

Pharma Companies
When small, mid-size, and top 50 pharma companies want to effectively train the right people, ensure compliance, and build strategic, long-lasting outsourcing partnerships – they turn to us. Being an extension of your team, we’ll stimulate new growth from the inside out.
CROs
Clinical Resource Organizations of any size are seeking the same thing: success. Successful patient and interim team member. Successful partnerships. Successful contracts. Successful teams. Successful trials. Successful sales. We help you get there.
Medical Device Companies
Managing clinical studies, compliance, training, expanding abroad – whatever your medical device company needs to reach the next level, you can depend on us to guide you through every step of the process.
Addressing Your Pain Points as a Biotech or Pharma Sponsor
Accelerating Your Journey to Market
You face a unique set of challenges that can derail your clinical trial if not managed effectively. We understand the high stakes and the pressure to deliver results on tight timelines and limited budgets. Many sponsors only realize they need expert support when things have already gone off track. Don’t wait until it’s too late. Here’s how we tackle the core you face:
- NVendor Oversight Complexity: Managing multiple vendors leads to misalignment and delays.
- NBudget Volatility: Uncontrolled costs jeopardize your trial’s success.
Solution: We implement a rigorous framework to manage vendor selection, contracting, and performance, ensuring alignment with your trial’s goals.
Solution: Our financial oversight includes detailed forecasting, real-time tracking, and vendor negotiations to prevent overruns.
- NRegulatory Compliance Risks: Navigating global regulations is complex and risky.
- NOperational Inefficiencies: Inefficiencies risk delaying critical milestones.
Solution: We ensure compliance through quality systems and readiness assessments, keeping your trial on track with all regulatory requirements.
Solution: We streamline operations with clear governance and KPIs, reducing delays and ensuring smooth execution.
- NTrial Failure Risk: Multiple dependencies increase the risk of failure.
- NManaging Digital Data Integrity: Ensuring accuracy and regulatory compliance for digital endpoints.
Solution: Expert guidance that helps you to mitigate risks by managing vendors, budgets, and compliance at every stage.
Solution: stringent data management protocols with digital vendors to guarantee data integrity and security.
Our Unique Approach to Clinical Development Success
A successful clinical trial is no accident—it’s the result of a structured, hands-on approach. We guide sponsors like you through every critical inflection point. These pivotal moments, where decisions or challenges arise, demand precise management to ensure trial success. We ensure that from vendor selection through trial completion, every phase is expertly navigated, risks are minimized, and performance is optimized.

Pharma Companies
When small, mid-size, and top 50 pharma companies want to effectively train the right people, ensure compliance, and build strategic, long-lasting outsourcing partnerships – they turn to us. Being an extension of your team, we’ll stimulate new growth from the inside out.
CROs
Clinical Resource Organizations of any size are seeking the same thing: success. Successful patient and interim team member. Successful partnerships. Successful contracts. Successful teams. Successful trials. Successful sales. We help you get there.
Medical Device Companies
Managing clinical studies, compliance, training, expanding abroad – whatever your medical device company needs to reach the next level, you can depend on us to guide you through every step of the process.
What is the Vendor Relationship Maximization Method (VRMM)?
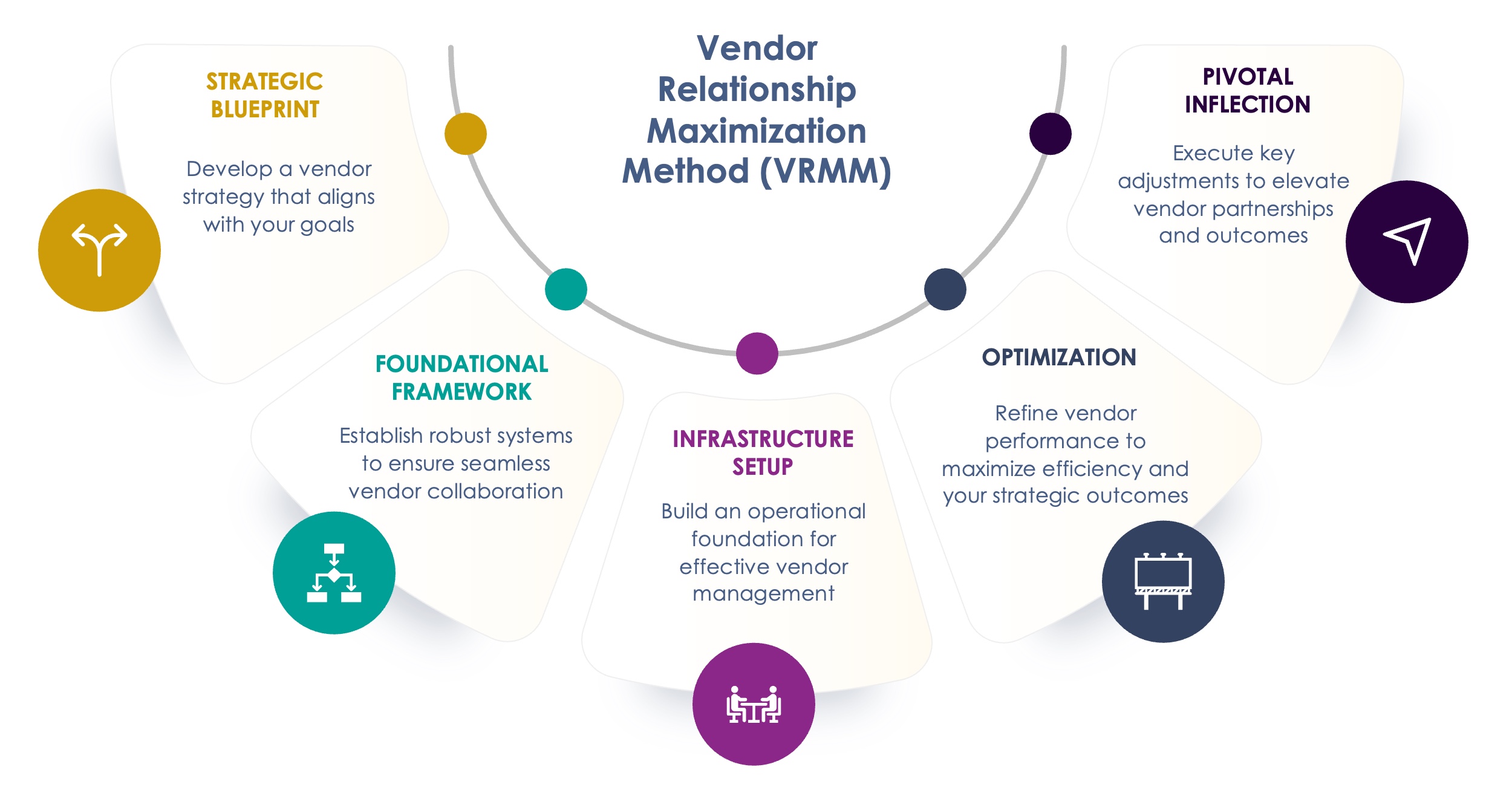
Over the last 13 years, we’ve developed a unique 5-step approach, the Vendor Relationship Maximization Method (VRMM), that focuses on optimizing sponsor-vendor relationships throughout the clinical development process.
- Strategic Blueprint: Vendor market scan, cost/timeline evaluation, and QMS assessment to set a solid foundation.
- Foundational Framework: Vendor selection, contract negotiation, and compliance checks to ensure trial readiness.
- Infrastructure Setup: Governance structures, risk management, and KPIs to establish a strong operational base.
- Optimization: Managing vendor performance, budgets, and collaboration to keep trials running efficiently.
- Pivotal Inflection: Inspection readiness, financial reconciliations, and regulatory submission support.
Pharma Companies
When small, mid-size, and top 50 pharma companies want to effectively train the right people, ensure compliance, and build strategic, long-lasting outsourcing partnerships – they turn to us. Being an extension of your team, we’ll stimulate new growth from the inside out.
CROs
Clinical Resource Organizations of any size are seeking the same thing: success. Successful patient and interim team member. Successful partnerships. Successful contracts. Successful teams. Successful trials. Successful sales. We help you get there.
Medical Device Companies
Managing clinical studies, compliance, training, expanding abroad – whatever your medical device company needs to reach the next level, you can depend on us to guide you through every step of the process.
Why Sponsors choose Seuss+
Expert guidance from the start, ensuring your trial runs smoothly—from vendor management to financial oversight and regulatory compliance. Let us take the operational and regulatory burdens off your shoulders so you can focus on the science.
- Hands-On Support: We don’t just consult—we partner with you as an extension of your team, taking on day-to-day responsibilities for managing vendors, budgets, and compliance. This hands-on approach ensures nothing slips through the cracks.
- Expertise Across All Phases of Clinical Trials: With over 13 years of experience, we’ve supported sponsors at every stage of the clinical trial process. Whether you’re in early-phase development or managing large-scale, global trials, we have the expertise to guide you through each critical inflection point.
- Global Reach, Personalized Service: Our global expertise allows us to support sponsors running international trials, but our personalized approach ensures you get the dedicated attention your trial deserves. We tailor our services to fit your unique needs and challenges.

Pharma Companies
When small, mid-size, and top 50 pharma companies want to effectively train the right people, ensure compliance, and build strategic, long-lasting outsourcing partnerships – they turn to us. Being an extension of your team, we’ll stimulate new growth from the inside out.
CROs
Clinical Resource Organizations of any size are seeking the same thing: success. Successful patient and interim team member. Successful partnerships. Successful contracts. Successful teams. Successful trials. Successful sales. We help you get there.
Medical Device Companies
Managing clinical studies, compliance, training, expanding abroad – whatever your medical device company needs to reach the next level, you can depend on us to guide you through every step of the process.
Therapeutic Areas Expertise
Gastroenterology
-
Chronic inflammatory bowel disease (IBD)
severe rare conditions with high unmet medical need (Short Bowel Syndrome (SBS))
Immunology
- GvHD
Rare diseases
- Achondroplasia (dwarfism)
- Lysosomal Storage Disorders
- TSC
Oncology
- Oncology
- Advanced solid tumors
- Acute Myeloid Leukemia (AML)
Rheumatology
- Ankylosing spondylitis (inflammatory
- Achondroplasia (dwarfism)
- Psoriatic Arthritis
- Osteoarthritis
Dermatology
- Psoriasis
- Hidradenitis suppurativa (rare disease dermatology)
- Psoriatic Arthritis
- Chronic hand eczema
- Pruritus
-
üAtopic dermatitis
Neurology
- Orphan conditions CNS
- Huntington’s Disease
- ALS
Hematology
- Hemophilia (Gene Therapy)
Ophthalmology
- Macular degeneration (retinal)
- Retinitis Pigmentosa
Respiratory
- Asthma
FAQs Who are Seuss+, And Who Do They Work With?
What does Seuss+ do?
Seuss+ provides hands-on, expert support to biotech and pharma sponsors, with a core focus on optimizing and maximizing sponsor-vendor relationships. We specialize in vendor management, risk mitigation, budget oversight, and regulatory compliance, ensuring seamless coordination throughout the clinical trial process. By actively managing vendor performance and aligning them with your goals, we help you get the most value from your partnerships. Whether it’s managing complex international trials, integrating digital endpoints, or navigating regulatory frameworks, we streamline operations, reduce risks, and drive your trial toward successful outcomes.
Are Seuss+ consultants?
While Seuss+ offers expert consulting services, we go far beyond traditional consulting. We are hands-on partners, fully embedded in the day-to-day execution of your clinical trial. Instead of simply offering advice, we take active responsibility for managing vendors, mitigating risks, overseeing budgets, and ensuring regulatory compliance. Our focus is on driving results and ensuring that every phase of your trial runs smoothly. By working as an extension of your team, we provide the strategic and operational support necessary to ensure your trial’s success.
How does Seuss+ enhance sponsor-vendor relationships?
At Seuss+, we focus on optimizing and maximizing the value of sponsor-vendor relationships to ensure that your clinical trial runs smoothly and efficiently. We actively manage every stage of the vendor relationship—from selection and contract negotiation to performance monitoring and risk mitigation. By fostering clear communication, setting measurable performance benchmarks, and aligning vendor activities with your clinical objectives, we create an environment where vendors are not just service providers, but strategic partners in your success. Our proactive oversight helps prevent misalignment, delays, and inefficiencies, allowing you to extract maximum value from each vendor while minimizing risks and costs.
Can Seuss+ support international clinical trials?
Absolutely. We are experienced in managing the complexities of global trials, from ensuring compliance with international regulations to coordinating multi-country vendor relationships, all while keeping your trial on schedule and aligned with regional requirements.
How does Seuss+ help prevent budget overruns?
We provide real-time financial tracking and active budget oversight. By continuously monitoring and optimizing resource allocation, we ensure that your trial stays on budget and that any financial risks are addressed proactively.
How does Seuss+ handle compliance across multiple regulatory frameworks?
Our team is deeply familiar with global regulatory requirements and continuously monitors changes in the industry. We ensure your trial remains audit-ready and compliant with standards set by agencies such as the FDA, EMA, and other regulatory bodies, no matter where your trial operates.
Schedule a consultation now
Let us help you

