Strategic Vendor Oversight and Trial Support for a Pivotal Phase 3 CNS Program
Summary
A small biotech company preparing for a pivotal Phase 3 trial in a challenging CNS indication had selected a CRO with deep therapeutic insight but limited experience in complex global delivery.
The sponsor’s internal team was lean, with functional gaps in oversight, vendor coordination, and contract governance. When COVID-19 introduced added risk, the need for hands-on support became urgent.
Seuss+ was brought in first to review a contract, then rapidly engaged to deliver fully integrated support across the trial lifecycle.
I cannot imagine NOT having Seuss+ as part of our business, to be honest. For a scaling company such as ours, there are vendors who can give you every piece. But being able to maintain the relationships and understanding of our company, and provide solutions, is something that Seuss+ excels in.”
ahead on trial enrollment milestone
oversight
on vendor documentation
What Seuss+ Delivered
Integrated Execution Support Across Workstreams
We served as an embedded partner across multiple workstreams, reinforcing clinical operations with structure, vendor accountability, and risk governance. Our support aligned tightly with the VRMM framework, ensuring momentum and regulatory confidence from execution to submission.
The Risk/Challenge
Selected CRO lacked global trial management experience
Limited internal capacity for governance, risk, and financial tracking
Gaps in oversight between CRO and its subcontractors threatened data quality
Trial timelines and communication strained by COVID-19 disruptions
The Results
Trial enrollment completed six weeks ahead of milestone target
Oversight gap closed, avoided data integrity risk between vendors
Operational alignment preserved during high-pressure vendor escalation
Client board maintained confidence through clear governance structure
Zero findings on vendor documentation during a regulatory inspection
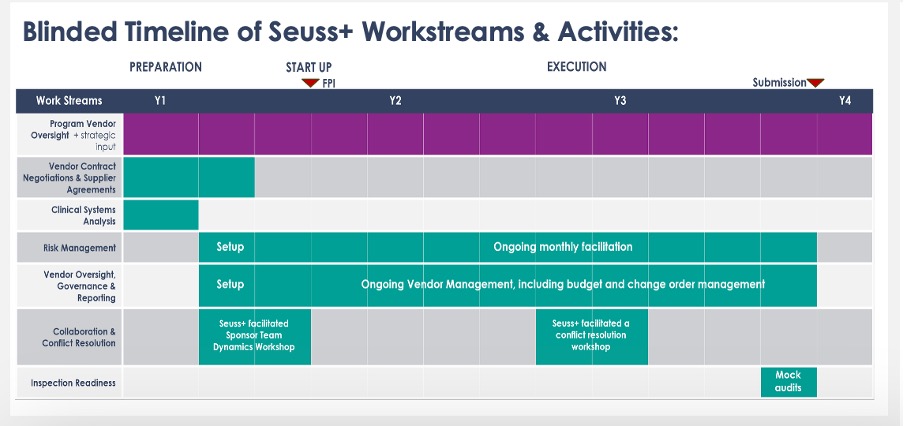
Workstream Deployment Timeline: From Risk Mitigation to Submission Success
How We Helped
Conducted risk assessment and embedded monthly issue resolution cadence
Facilitated a structured conflict resolution workshop during vendor escalation
Managed quarterly governance meetings, focused on KPIs and delivery health
Implemented budget tracking tools for financial transparency and reporting
Prepared documentation and processes for inspection-readiness
Other Related Case Studies
Schedule a consultation now